Episode 3: The Dating Game
This episode will finish our trilogy of introductions to geology before we start exploring Earth’s history from the beginning. There are many more basic concepts we will learn about as they start to appear in the Precambrian, so don’t worry that we haven’t covered topics like plate tectonics yet. We’ll get there, I promise.
In Episode 1, I introduced the Earth Calendar, a streamlined view of the planet’s past, compressing 4.6 billion years into 365 days.
But how do we know that the Earth is 4.6 billion years old? How do we know how old any rock is?
Today, we will answer those questions by taking a brief look at geochronology, the science of dating Earth’s ancient past. After this episode, when you hear a scientist, a professor, (or a podcaster) tell you that a rock is 3 billion years old, you will know exactly how that number was calculated. These dates are not pulled out of thin air- quite the opposite. At the end of the episode, we’ll return to the Earth Calendar armed with the confidence of dates, and I’ll give a brief outline of the Podcast’s future.
Humans have invented many ways to keep track of time, from sundials to smartphones. Of all these inventions, the best analogy for rocks as timepieces is the hourglass. If you have an hourglass, pause the episode and bring it out of the attic or basement. If you have a really fancy one, take a picture and send it to bedrock.mailbox@gmail.com. If not, just imagine one, or watch the intro to Days of Our Lives- tell your friends it’s for science.
An hourglass is made of two essential parts: the glass container and the sand within. When you flip over the hourglass, sand will trickle down at a regular pace from the upper to lower chambers. Keep this image in mind, or in hand, as we go forward. Rocks aren’t literal hourglasses- they don’t reset when you flip them upside down. But the two aspects of an hourglass- a sturdy container holding something undergoing constant steady change, are essential to calculate any geologic date. We will start by looking at the “sand” in our analogy, the atoms undergoing consistent change, then zoom out to the minerals that preserve these changes, the “containers” if you will. When you’re ready to begin, let’s turn over the hourglass.
Part 1: The Radioactive Clock
Any timepiece of ancient Earth needs both consistency and longevity. An old clock that slows down and needs to be wound up is not consistent, and eventually gives you the wrong time. In contrast, the sand in an hourglass falls at a constant rate, but will run out after a few minutes.
So what natural material changes predictably over billions of years? What will be the consistent, slow-moving sand in our hourglass of deep time? The answer is on a much smaller scale: atoms.
Many atoms are stable- they have reached a zen-like balance in their cores between protons and neutrons, the building blocks of their nuclei. This balance can be disrupted, but it takes some effort. But some atoms are less balanced- their cores have many more neutrons than protons, and the atoms become more unstable. Just looking at these unstable atoms is enough to set them off, but this doesn’t happen immediately, and they don’t simply disappear into a poof of smoke. Instead, unstable atoms decay into more stable atoms.
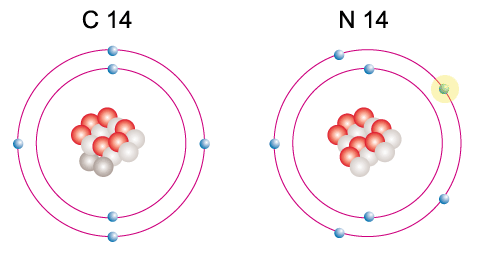
Radiocarbon & nitrogen-14. Spot the three differences between them. Though very similar, N-14 is more stable.
A famous example is radiocarbon, or carbon-14. Radiocarbon is a small atom, but it has 8 neutrons vs 6 protons, a decent imbalance for its petite frame. Instead of immediately shoving off these pesky neutrons and getting on with its day, radiocarbon holds onto them like Midwestern resentment. Eventually, the carbon will have a really bad day and spontaneously decay into nitrogen-14, reaching a zen balance of 7 protons and 7 neutrons. For a radiocarbon atom, the awkward period of imbalance beforehand can last anywhere from days to thousands of years.
Let’s pause. A minute ago, I said that that a geological clock needs consistency. But if a radiocarbon atom can decay whenever it feels like, how can that form the basis for a consistent timepiece? That is a good question, one that bugged me for a while in my chemistry classes. Fortunately, there is an easy answer.
Geologists cannot use individual atoms to measure time. Instead, we measure the chemistry of an entire rock with thousands of unstable atoms. Let’s return to the hourglass. Inside are thousands of sand grains, but if I only focus on an individual grain, I lose the larger picture. Some grains will immediately fall through as I tip the hourglass over, while others will take minutes to drop. Does that mean the timepiece is broken? No, just that the rate of change is recorded by many sand grains falling from one state to another. The same concept is applied to rocks by measuring the amount of unstable atoms like radiocarbon to their stable descendants like nitrogen-14.
Djoser’s Pyramid (~4680 years old), one of the first places to be dated using radiocarbon. PS- I’ve already broken my promise not to mention pyramids.
All of the radiocarbon in a rock will decay to nitrogen after 50,000 years, the end of the hourglass. This is nowhere near the age of the Earth, but I’m starting with radiocarbon because it is the most well-known method in geochronology. You will hear more news stories about radiocarbon than all the other techniques combined. That’s because the decay of radiocarbon beautifully overlaps with human history. The first radiocarbon measurements were taken from the tombs of early Egyptian pharaohs, more than four thousand years old. Archeologists already knew how old these tombs were from written records, but the chemistry confirmed it. Since then, radiocarbon dates have been confirmed by other independent clocks such as tree rings.
OK, so radiometric dating works for carbon, we have outside sources like trees and temples to confirm that. How can we be so sure for atomic clocks that last over billions of years?
Fortunately, carbon is not alone in its instability. There are hundreds of unstable atoms that decay within years, days, milliseconds, and even shorter. All of them follow the same pattern of decay: the half-life.
To explain the half-life, I’d like you to perform an experiment. All you’ll need is a piece of notebook paper, a pencil, and a timepiece- pause the episode now if you want to get these things. Ready? Every five seconds, take the piece of paper and tear it in half. Mark one half with an x and put it aside. After each tear and mark, you should only have a blank piece in your hand. Keep repeating until you can’t tear the paper apart with your fingers anymore. For a regular piece of paper, the limit should be around 10 tears. Don’t forget to recycle it!
This paper had a half-life of five seconds- no matter how much there was, half was gone in five seconds. The unstable element francium-232 also has a half-life of five seconds, which has been measured by humans in laboratories. Radiocarbon has a half-life around 5,000 years. For every increment of time you can think of, there is an unstable element that decays over that timeframe. Each and every one follows the same half-life rule you just applied to that poor piece of paper. The atomic half-life is a well-founded idea, confirmed by thousands of laboratory experiments and real-world applications since 1907. Even if an element decays over billions of years, it still decays, and we can use that tiny amount of change to calculate a half-life.
Nearly every date given in this podcast, including the 4.6 billion years of Earth’s age, was calculated by measuring the decay of uranium into lead. Two uranium isotopes are used as geologic clocks- one has a half-life around 700 million years, while the other’s half-life is 4.5 billion years, just different from the age of the Earth. When measured together, these two uranium-lead clocks provide a useful check on each other, like two timers at race. If one is off, more work needs to be done.
So now we know that if you gather enough unstable uranium atoms, they will predictably decay into lead over billions of years. The concept of radiometric dating has been successfully used for nearly a century, and is one of the cornerstones of modern geology. Now let’s see how the uranium clock is preserved in the rocks themselves.
Part 2: The Crystal Time Capsule
Zircon crystal (red) embedded in calcite, Gilgit, Pakistan (~1 cm)
Just as sand requires a vessel to make an hourglass, uranium atoms need to be contained within a mineral to make a geological clock. But not just any crystal will do. The perfect mineral needs to be durable to resist billions of years of metamorphic heat and pressure. A time-keeping mineral also needs to be common- the more crystals we analyze, the more detailed Earth’s timeline becomes.
Fortunately, just such a mineral exists: its name is zircon. That name might ring a bell, especially for newlyweds. You might be familiar with cubic zirconia, the artificially-made material in synthetic “diamond” rings. The natural mineral zircon is slightly different, but both are incredibly tough. Zircon is also a fairly common mineral, but some rocks have more zircon than others. To see which rocks have the most zircon, we need to revisit a location from last episode: the magma chamber.
Zircon is one of many minerals that form as magma chambers slowly cool into granite. You are unlikely to see them in your kitchen countertops, though- zircons are usually less than a millimeter long. Despite their size, zircons can still hold enough uranium atoms to form useful timepieces. If we zoom in even further into the atomic structure of a single zircon, we will see one final property that makes the mineral an excellent timepiece.
Crystal lattice of zircon, showing patterns of oxygen, zirconium, and silicon
You can imagine the microscopic structure of a zircon like an old-school jungle gym made of steel pipes, with each intersection holding an atom of silicon, oxygen or zirconium, separated by wider empty spaces. Geologists call this structure a “crystal lattice”. The spaces in the lattice can sometimes trap other atoms as the crystal grows, like a ball stuck inside the jungle gym. The shape and chemistry of zircon easily traps uranium atoms, but lead simply cannot fit. Remember, uranium decays into lead over time. If zircon crystals formed with both uranium and lead stuck inside, we would measure an incorrect date- it would be like starting an hourglass when there is sand in each chamber. You wouldn’t know exactly how much time had passed.
Because zircons are born inside magma chambers, you are most likely to find them in granite and other intrusive rocks. Dates measured from these zircons will tell you when the magma chamber cooled. But granites aren’t the only rocks that hold zircons. When granites are eventually exposed on Earth’s surface, the constant chisel of water and wind will grind the rocks into sand. This is where the tiny zircon’s durability comes in handy- these crystals were forged above 800 C, they can handle a little bath. Zircons are plucked out of granites, tossed around by rivers or waves, and eventually settle down on the beach into sandstones. We call these transported crystals “detrital zircons”, from the same word as detritus. This migration does not reset the clock- the zircon still records the first date it cooled underground, not the date it relocated on the beach. So while zircon dates from granites are more exact, dates from sandstones can tell us interesting stories about sediment transport on ancient Earth.
To recap: Zircons are durable, common, and are naturally shaped to hold uranium atoms. These atoms turn into lead at a known, persistent rate- the half-life. These properties make zircon the standard mineral for measuring Earth’s ancient past, and one of the most important minerals in geology. This is coming from someone who has never worked with zircons. Many people ask me how I date the rocks I study, and I tell them…
I don’t.
Most geologists don’t personally measure the dates of their rock samples. The process to sift zircons and measure uranium concentrations is long and complicated, perfected by specialists called geochronologists. Every date from Earth’s ancient past, every number in the millions and billions, was measured by a geochronologist. They would tell you that there are many other elemental clocks and minerals that I have completely ignored, and they would be right- we will take those as they come. In fact, we’ll learn about one next episode. I also hope to interview several geochronologists in future episodes- they will expand this brief summary more than I ever could.
Part 3: Going Forward
Here’s what we’ve learned in these first three episodes.
Earth is 4.6 billion years old. This age, and many others, has been calculated by measuring changes in unstable atoms trapped inside incredibly tough crystals- the hourglasses of deep time.
Earth is 4.6 billion years old, and for nearly 90% of that history the planet was an incredibly alien world, but certainly not a dead one. The stories of this Precambrian Era are set in stone, stories of molten rock, stories of water and life, and stories of metamorphosis.
Earth is 4.6 billion years old, but the number of a date is less important than the event itself and its context. When you look back at an old photograph, you might not remember the exact time or even year it was taken, but hopefully you remember the story behind it.
To wrap up this episode, here’s my roadmap for the months ahead. This podcast will be divided into eight seasons, one for each major division of the Precambrian.
Season 1, starting next episode, will cover the Hadean, Earth’s earliest days from 4.6 to 4 billion years ago, Jan to Feb on the Earth Calendar. In the Hadean we will see the Earth form, cool down, and nearly be destroyed at the very beginning of its history. Seasons 2-5 will showcase the Archean, 4 to 2.5 billion years ago, March to June. Here we will witness the growth of continents, the first evidence of life, and eventually the first breath of oxygen. Finally, Seasons 6-8 will conclude with the Proterozoic, 2.5 to 0.5 billion years ago, July to November. Here, oxygen really starts to change Earth’s chemistry, the planet will freeze over multiple times, and at the very end, the first animals will evolve.
One final note- as we grow comfortable using billion-year clocks, I will use the Earth Calendar more sparingly, so I’m not constantly listing two dates. Also, if you’re listening live, you’ll notice that the podcast is starting in January 2022- you might think : “So, will we finish everything by November?” Absolutely not. This show will vary between weekly and bi-weekly based on my schedule, and will certainly take more than a year to finish.
I do not know when the story will end, but it’s time we start at the beginning, in the Hadean, 4.6 billion years ago, in the Cradle of Stardust.
***
Thank you for listening to Bedrock, a part of Being Giants Media. As the show takes off, I would love to hear your input on style, topics, and people to interview- you can drop me a line at bedrock.mailbox@gmail.com. See you next time.
Images:
Hourglass icon: halfwitty
https://commons.m.wikimedia.org/wiki/File:Hourglass_icon.png
Radiocarbon & N-14: Aloha2009
https://commons.wikimedia.org/wiki/File:N14-C14.gif
Djoser’s pyramid: Charles Sharp
https://commons.wikimedia.org/wiki/File:Saqqara_pyramid_ver_2.jpg
Torn paper: MaxPixel
https://www.maxpixel.net/Torn-Paper-Torn-Paper-Edge-Paper-Ripped-Torn-1816679
Zircon crystal:Robert Lavinsky
https://commons.wikimedia.org/wiki/File:Zircon-dtn1a.jpg
Zircon crystal lattice: Materialscientist
https://commons.wikimedia.org/wiki/File:Zircon.GIF
Earth Calendar: Dylan Wilmeth
Music:
Taking it In by Michael Brandon
The Goldberg Variations: No. 3 by Johann Sebastian Bach, performed by Kimiko Ishizaka https://commons.wikimedia.org/wiki/File:Goldberg_Variations_04_Variatio_3_a_1_Clav._Canone_all%27Unisuono.ogg
The Goldberg Variations: No. 5 by Johann Sebastian Bach, performed by Kimiko Ishizaka
https://commons.wikimedia.org/wiki/File:Goldberg_Variations_06_Variatio_5_a_1_ovvero_2_Clav.ogg
The Carnival of the Animals: Hémiones by Camille Saint-Saens, performed by Neil and Nancy O’Doan https://commons.wikimedia.org/wiki/File:Saint-Saens_-_The_Carnival_of_the_Animals_-_03_Hémiones_(animaux_véloces).ogg
Auld Lang Syne (traditional) performed by Starlifter and Roots in Blue
https://commons.wikimedia.org/wiki/File:Auld_Land_Syne_(2020)_-_Starlifter_and_Roots_in_Blue_-_United_States_Air_Force_Band_of_Mid-America.mp3